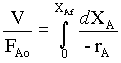
The following conversion data were obtained in a tubular flow reactor for the gas-phase pyrolysis of acetone at 520oC and 1 atm.
CH3COCH3 -> CH3=C=O + CH4
Flow rate, g/hr 130 50 21 10.8 Conversion of acetone 0.05 0.13 0.24 0.35
The reactor was 80 cm long with 3.3 cm inner diameter. What rate equation is suggested?
Calculations:
Volume of reactor = (π/4)D2 L = 684 cm3 = 0.684 lit
Molecular weight of acetone = 12 + 3 + 12 + 16 + 12 + 3 = 58
The above flow rate data in g/hr are converted into gmol/hr, by dividing them with Molecular weight.
Flow rate, g/hr 130 50 21 10.8
Molal flow rate, gmol/hr (FAo) 2.2414 0.8621 0.3621 0.1862
Conversion of acetone (XA) 0.05 0.13 0.24 0.35
For the (tubular flow) plug flow reactor the design equation is,
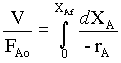
→ 1
CAo = pAo/RT = 1.01325 x 10-5/(8314 x (273 + 520))
= 0.01537 kmol/m3 = 0.01537 gmol/lit
For the first order reaction, (variable density systems)
-rA = kCA = kCAo(1 - XA)/(1 + εAXA) → 2
substituting for -rA from equn.2 in equn.1,
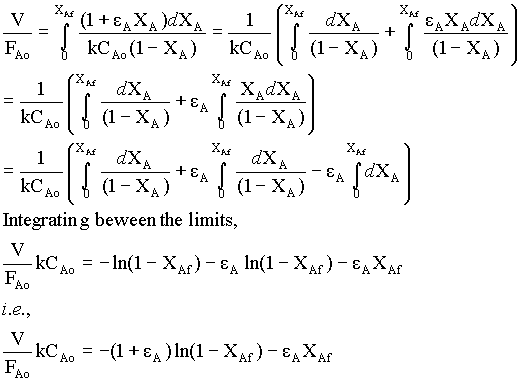
for the given gas-phase reaction,
εA = (VXA = 1 - VXA = 0) / VXA = 0 = (2 - 1)/1 = 1Therefore,
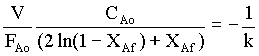
For the constant V and CAo,
FAo ( 2 ln(1 - XAf) + XAf) = -VCAok = constant.
→ 3For second order reaction, (by Analytical integration)
k CAo2 V/FAo = 2
εA(1 + εA) ln(1- XAf) + εA2XAf + (εA + 1)2 XAf/(1 + XAf)For the given problem, εA = 1. Therefore,
(4 ln(1- XAf) + XAf + 4 XAf/(1 + XAf) ) FAo = k CAo2 V = constant. → 4
We will check the above relations for various data available.
|
Molal flow rate, gmol/hr (FAo) |
2.2414 |
0.8621 |
0.3621 |
0.1862 |
|
Conversion of acetone (XAf) |
0.05 |
0.13 |
0.24 |
0.35 |
|
For First order: FAo ( 2 ln(1 - XAf) + XAf) |
-0.11787 |
-0.12804 |
-0.11184 |
-0.09525 |
|
For Second order: (4 ln(1- XAf) + XAf + 4 XAf/(1 + XAf) ) FAo |
0.124069 |
0.14712 |
0.146799 |
0.145369 |
From the tabulated data, it could be seen that the second order mechanism is well fitting the data, than first order.
Therefore, the reaction is following second order.
k CAo2 V = average of
(4 ln(1- XAf) + XAf + 4 XAf/(1 + XAf) ) FAo for various FAo valuesk CAo2 V = 0.146429 (
obtained by omitting the first data)Therefore, k = 0.146429 / (0.015372 x 0.684) = 906.2 lit/(gmol.hr)
And the equation is,
-rA = 906.2 CA2 gmol/(lit.hr)
Instead of analytical integration for 2nd order reaction, we can use numerical integration (Simpson's 1/3 rule)
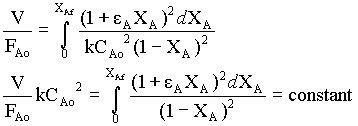
Numerical integration is obtained for the function
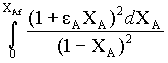
by taking 10 intervals.
|
Numerical Integration - by Simpson's rule |
|||||||||||
|
0.05 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|
0 |
0.005 |
0.01 |
0.015 |
0.02 |
0.025 |
0.03 |
0.035 |
0.04 |
0.045 |
0.05 |
|
|
1 |
1.0202 |
1.0408 |
1.0618 |
1.0833 |
1.1052 |
1.1275 |
1.1503 |
1.1736 |
1.1974 |
1.2216 |
|
|
0.0554 |
|||||||||||
|
0.13 |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
0 |
0.013 |
0.026 |
0.039 |
0.052 |
0.065 |
0.078 |
0.091 |
0.104 |
0.117 |
0.13 |
|
|
1 |
1.0534 |
1.1096 |
1.1689 |
1.2314 |
1.2974 |
1.3670 |
1.4405 |
1.5182 |
1.6002 |
1.6870 |
|
|
0.1707 |
|||||||||||
|
0.24 |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
0 |
0.024 |
0.048 |
0.072 |
0.096 |
0.12 |
0.144 |
0.168 |
0.192 |
0.216 |
0.24 |
|
|
1 |
1.1008 |
1.2118 |
1.3344 |
1.4699 |
1.6198 |
1.7861 |
1.9708 |
2.1764 |
2.4057 |
2.6620 |
|
|
0.4054 |
|||||||||||
|
0.35 |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
0 |
0.035 |
0.07 |
0.105 |
0.14 |
0.175 |
0.21 |
0.245 |
0.28 |
0.315 |
0.35 |
|
|
1 |
1.1503 |
1.3237 |
1.5243 |
1.7572 |
2.0285 |
2.3459 |
2.7192 |
3.1605 |
3.6853 |
4.3136 |
|
|
0.7807 |
|||||||||||
|
XA |
Area |
FAo |
FAo XA Area |
|
0.05 |
0.0554 |
2.2414 |
0.1241 |
|
0.13 |
0.1707 |
0.8621 |
0.1471 |
|
0.24 |
0.4054 |
0.3621 |
0.1468 |
|
0.35 |
0.7807 |
0.1862 |
0.1454 |
Last Modified on: 30-Apr-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in