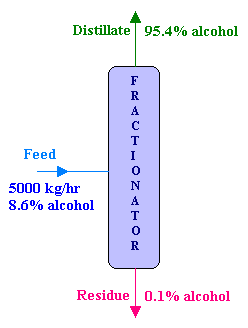
A solution of ethyl alcohol containing 8.6% alcohol by weight is fed at the rate of 5000 kg/hr to a continuous fractionating column operating at atmospheric pressure. The distillate which is the desired product contains 95.4% alcohol by weight and the residue from the bottom of the column contains 0.1% alcohol by weight. Calculate the following:
Calculations:

Overall material balance:
Feed = Distillate + Residue
i.e., F = D + R
D + R = 5000 → 1
Component balance for Alcohol:
FxF = DxD + RxR
Where x 's are the mole fraction of alcohol in various streams.
0.954 D + 0.001 R = 5000 x 0.086 = 430 → 2
Equations 1 and 2 contains 2 unknowns D and R, and that can be solved as follows:
Multiplying equation 1 by 0.001,
0.001D + 0.001 R = 5 → 3
subtracting equation 3 from equation 2,
0.953 D = 425
D = 446 kg/hr
i.e.,
Distillate flow rate = 446 kg/hrTherefore, R = 5000 - 446 = 4554 kg/hr
i.e.,
Residue flow rate = 4554 kg/hrEthanol leaving in the residue (i.e., ethanol lost) = 4554 x 0.001 = 4.554 kg/hr
Ethanol entering in the feed = 5000 x 0.086 = 430 kg/hr
Percentage loss of ethanol
= 100 x (4.554 / 430) = 1.06Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in