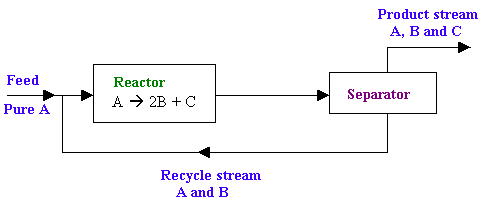
The reaction A -> 2B + C takes place in a catalytic reactor (diagram is given below). The reactor effluent is sent to a separator. The overall conversion of A is 95%. The product stream from the separator consists of B, C and 0.5% of A entering the separator, while the recycle stream consists of the remainder of the unreacted A and 1% of B entering the separator. Calculate the

Calculations:
Basis: 1 mole of pure A in the feed
From the reaction, A → 2B + C
1 mole of A produces 2 moles of B and 1 mole of C for complete conversion.
From the problem statement, overall conversion is 95%. Therefore, 1 mole of feed A will produce
2 x 0.95 mole of B ,
1 x 0.95 mole of C and the
unreacted A is 0.05 mole.
i.e.,
Product stream contains:
A : 0.05 mole
B : 1.9 mole
C : 0.95 mole
Material Balance for the compound B:
From the problem statement, B in the recycle stream = 1% of B entering the separator.
Therefore, B in the product stream = 99% of B entering the separator = 1.9 mole
Total B entering the separator = 1.9/0.99 = 1.919 mole
And, amount of B in the recycle stream = 1.919 x 0.01 = 0.019 mole.
Material Balance for the compound A:
From the problem statement, A in the product stream = 0.5% of A entering the separator.
i.e., A in the product stream = 0.5% of A entering the separator = 0.05 mole.
Therefore, amount of A entering the separator = 0.05/0.005 = 10 mole.
And, the amount of A in the recycle stream
= Amount of A entering the separator - Amount of A in the product stream
= 10 - 0.05 = 9.95 mole.
Amount of A entering the reactor = fresh A + recycle A = 1 + 9.95 = 10.95 mole.
Amount of A converted in the reactor = moles of A entering the reactor - moles of A entering the separator = 10.95 - 10 = 0.95 mole
Single pass conversion of A in the reactor
= 100 x Amount of A converted in the reactor / Amount of A entering the reactor
= 100 x (0.95/10.95) =
8.676%Total moles of recycle stream = moles of A in recycle stream + moles of B in recycle stream
= 9.95 + 0.019 = 9.969 mole
Molar ratio of recycle to feed
= 9.969/1 = 9.969Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in